This Recently Uplisted NYSE Stock Could Offer Investors the Best Way to Play this Rapidly Growing Market for Maximum Profit Potential
The next huge potential opportunity in the biotech sector has arrived.
This opportunity comes in the form of psychedelic mushrooms…which are now being shown in clinical studies worldwide to have significant potential in helping treat a wide range of mental health issues.
Here’s just how significant the market potential is in this area:
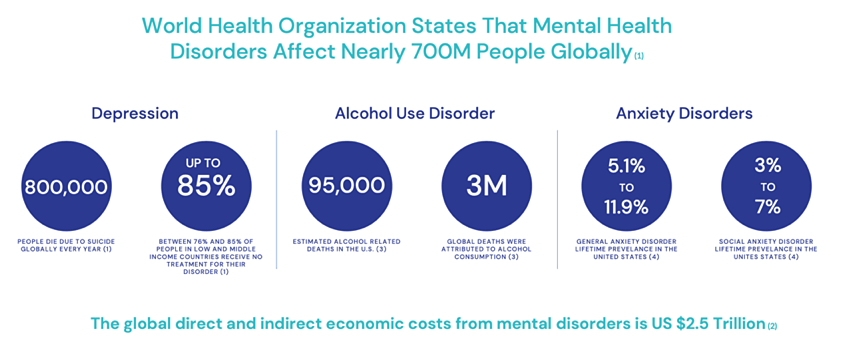
- More than 700 million people all over the world are now affected with some form of mental disorder, such as anxiety, depression, addiction, PTSD, eating disorders and more.
- And according to a recent survey, since the Covid-19 pandemic began, 46% of American workers are reported to be have been suffering from mental health issues – up from 39% just one year prior to the pandemic.[i]
- For the last several decades, those suffering from conditions like these were steered toward treatments that have been largely ineffective or have come with potentially dangerous side effects.
- On top of that, those ineffective treatments – and the impact of their success or failure – have come at a huge cost. How huge? Just recently, one study estimated the total economic impact at more than $2.5 trillion annually.[ii]
- Finally – thanks to the extraordinary potential of psychedelics – relief may be on the horizon for the hundreds of millions still suffering.
As this opportunity is unfolding – and the market growing quickly – one company has emerged as potentially having the highest upside for investors in this space:
That company is Cybin, Inc. (NYSE: CYBN).

Cybin has its sights set on revolutionizing mental healthcare and transforming the treatment landscape.
The company is focused on progressing psychedelic therapeutics by utilizing…
- Proprietary drug discovery platforms…
- Innovative drug delivery systems…
- And novel formulation approaches and treatment regimens for psychiatric disorders.
The company’s extraordinary efforts are coming at a time of unprecedented research activity in the psychedelic space.
In fact, the U.S. Food and Drug Administration has already granted “breakthrough status” to psilocybin therapies on multiple occasions.
This action is meant to accelerate the typically methodical process of dug development and review and is only granted when preliminary evidence suggests the therapy in question may be a significant improvement over existing therapies.
It is against this extremely research-friendly backdrop – with a number of psychedelic therapies in the advanced stages of FDA clinical trials – that Cybin is moving forward with its own potential breakthrough treatment development.
Cybin’s Active Drug Programs Are Targeting Multi-Billion Dollar Global Market Opportunities
Cybin, Inc. (NYSE: CYBN) currently has four active drug programs targeting a wide range of disorders:
- Major Depressive Disorder (CYB001) – The global market size for major depressive disorder was estimated at US $12.7 billion in 2020 and is expected to grow at a CAGR of around 3% from 2021 through 2026.
- Alcohol Use Disorder (CYB003) – The global market size for alcohol use disorder is projected to grow to US $1.49 billion – at a CAGR of 6.5% — by 2030. And the total treatment market for global drug addiction is expected to reach US $31.17 billion by 2027.
- Anxiety Disorders (CYB004) – The global market for anxiety disorders is projected to reach US $7.4 billion by 2023, growing at a CAGR of 2.5% over the next two years.
- and Therapy Resistant Psychiatric Disorders (CYB005) – targeting a wide range of conditions for which effective therapies have proven elusive to date.
As of this writing, the company has 14 patent filings covering: Novel psychedelic compounds, delivery mechanisms, supportive treatment platforms and a drug discovery pipeline of modified and novel tryptamines and phenethylamines.
Cybin’s CYB001, Targeting Major Depressive Disorder, Approved for Phase 2a Clinical Trial
Just recently, the company received approval to launch a Phase 2a clinical trial on psilocybin for patients suffering from major depressive disorders (MDD).
The head-to-head study will test Cybin’s proprietary sublingual psilocybin formulation against a 25mg psilocybin capsule on 40 patients.
This unique drug delivery platform works via a film that is loaded with psilocybin and placed under the tongue, where it is designed to deliver psilocybin quickly – and directly into the bloodstream – without having to go through the liver.
Results on this Phase 2a trial are expected by the end of the year, and positive results could put the company in a strong position with a critical first-mover advantage.

In addition to CYB001, the company’s other active drug pipeline programs are ongoing in the discovery and preclinical phases.
Cybin’s CYB003 and CYB004 are in late-stage preclinical trials and are proprietary psychedelic molecules that target Alcohol Use Disorder and Anxiety Disorder.
In the case of CYB004, targeting Anxiety Disorder, the company is planning to use as its delivery system an inhaler system that has already received regulatory approval.
Cybin Is Taking a Comprehensive Approach to Mental Wellness
 One of the things that makes Cybin, Inc. (NYSE: CYBN) unique is that the company’s 3-pillar development strategy.
One of the things that makes Cybin, Inc. (NYSE: CYBN) unique is that the company’s 3-pillar development strategy.
This comprehensive approach allows the company to enjoy potential first-mover advantages with not only its therapeutic candidates, but also with its drug delivery platforms and potential treatment regimens.
Here is a brief overview of Cybin’s 3-pillar development strategy:
Cybin’s Strategic Partnerships Help Improve the Company’s Drug Discovery Process
To help advance its own psychedelic drug research, Cybin, Inc. (NYSE: CYBN) has entered into a handful of critical strategic partnerships.
One such partnership is a research and development agreement – signed in early July – with Greenbrook TMS, a company that operates 129 outpatient mental health service centers in the United States.
Cybin’s partnership agreement allows the company to access Greenbrook TMS’s extensive patient base to advance clinical research on its compounds and to help recruit participants for upcoming trials.
Another partnership is Cybin’s agreement with Kernel, a brain imaging company.
Kernel’s wearable device – known as Kernel Flow – is a non-invasive brain interface that records real-time, cortical hemodynamics to establish precise patterns of brain activity.
This partnership will allow access to Kernel Flow and permit Cybin to potentially see real-time brain activity during a psychedelic treatment session, which could help the company better target these molecules in the future.
Cybin (NYSE: CYBN) Has a Leadership Team Loaded With Proven Business Builders and Great Scientific Minds
One of the most important considerations with any company involved with rapidly emerging technology is the quality of its leadership team.
Does the company have access to both the business-building expertise and scientific knowledge necessary for rapid growth?
In the case of Cybin (NYSE: CYBN) the answer is a resounding yes.
The company’s leadership team has deep rooted psychedelic, pharmaceutical, regulatory and academic research experience.

This team has…
- Successfully helped develop widely used drugs such as: Allegra, Sabril, Anzemet and Vaniqa.
- 300 combined peer reviewed publications by scientific leadership including work in addiction and psychedelics.
- Collectively been involved in 37 exits across the biotech sector and various other verticals.
- Overseen more than 60 IND programs with the Food & Drug Administration.
- Worked on the development for the first FDA approved psychedelic compound which is covered by healthcare insurance.
Bottom Line: Now is the Time to Consider Investing in Cybin (NYSE: CYBN)
The bottom line: Now is the time for you to seriously consider the opportunity presented by Cybin (NYSE: CYBN) while this company remains under the radar.
That’s because the company is not likely to remain beneath the radar for long.
In fact, Cybin is already being taken seriously in the marketplace as an emerging leader in the space, as the company has attained analyst coverage by 6 firms and is now included in 2 psychedelic ETFs.

Top 5 Reasons to Consider Cybin, Inc. (NYSE: CYBN) Right Now:
- Rapidly Growing Market – The market for psychedelic drugs is growing rapidly – at a CAGR (compound annual growth rate) of 13.3% — and is expected to reach $7.56 billion by 2028[iii]. That’s up from $2.82 billion in 2020 and is thanks in large part to the need for more effective treatments for the mental health conditions impacting so many all over the globe.
- Impressive Pipeline – Cybin (NYSE: CYBN) has an impressive pipeline of drugs in development for the treatment of a wide array of mental health conditions. This pipeline includes a proprietary formulation of psilocybin that is currently in phase 2 clinical trials.
- Unique 3-Pillar Development Strategy – Cybin’s approach is to deploy three development strategies including a novel drug discovery platform…a proprietary drug delivery platform…and a novel treatment regimen to help clinicians improve patient outcomes.
- Experienced Team – The company’s leadership team has deep rooted psychedelic, pharmaceutical, regulatory and academic research experience. This includes having successfully developed a number of widely used drags and over 300 combined peer reviewed publications by scientific leadership.
- Uplisted to NYSE – The company commenced trading on the New York Stock Exchange in August 2021, becoming the first psychedelics company on the NYSE. This validation by the NYSE for the company will help drive increased awareness for the company in the months ahead.
[ii] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5007565/
[iii] https://www.globenewswire.com/en/news-release/2021/07/14/2263133/0/en/Psychedelic-Drugs-Market-CAGR-at-13-3-with-Analysis-of-Growing-Technology-Trends-Industry-Research-Future-Growth-and-Size-Projection-by-2028.html





